Improve drug development speed
and safety
Monitor, manage and communicate with study participants at home to gain robust health insights.

The Current Health platform streamlines the capture, monitor, and analysis of participant health data using remote monitoring devices and supporting services.
Partnered with 5 of the top 10
pharmaceutical companies worldwide
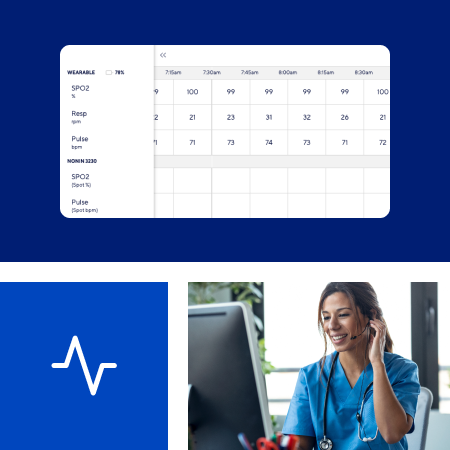
Continuous and intermittent monitoring
Leverage our wide portfolio of near real-time remote monitoring devices to capture the broadest picture of participant health, helping to support clinical safety monitoring and digital biomarker development.

Configurable dashboard and alarms
Enable highly specific and tailored alarms to stratify participant risk and identify signs of health deterioration or adverse events, with customizable escalation pathways for agreed interventions.
Sarah Cannon
Sarah Cannon moves more than 75% of their CAR-T therapies to the outpatient setting.
-
1,200+ bed days saved
-
17% of patients avoided hospitalization entirely
-
75% decrease in length of stay for those who had to be hospitalized
-
2-3 minute alarm response time

Increased data capture and
participant safety monitoring
to set up our kit
patient adherence
task adherance
patients engaged
Care at home for
high-risk oncology patients
How top cancer centers are delivering care at home for CAR-T and BiTE patients
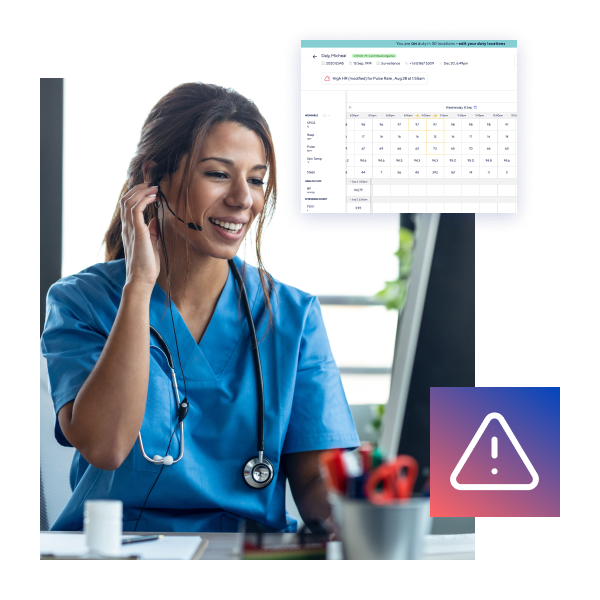
Our Caring Center team help to optimize participant care and reduce the burden on trial site teams with 24/7/365 clinical monitoring, providing expert triage, management, and agreed escalation, where required.
By leveraging Best Buy’s robust logistics infrastructure, we can operationalize clinical trials at scale, with unmatched efficiency.


Our clinical research team collaborate closely with our partners to enrich the collective evidence base for clinical trials and treatment therapies.
The comprehensive training and support we provide for sponsors and trial sites, helps to guarantee smooth and efficient trial operations with strategic program success management throughout.